2026-01-07
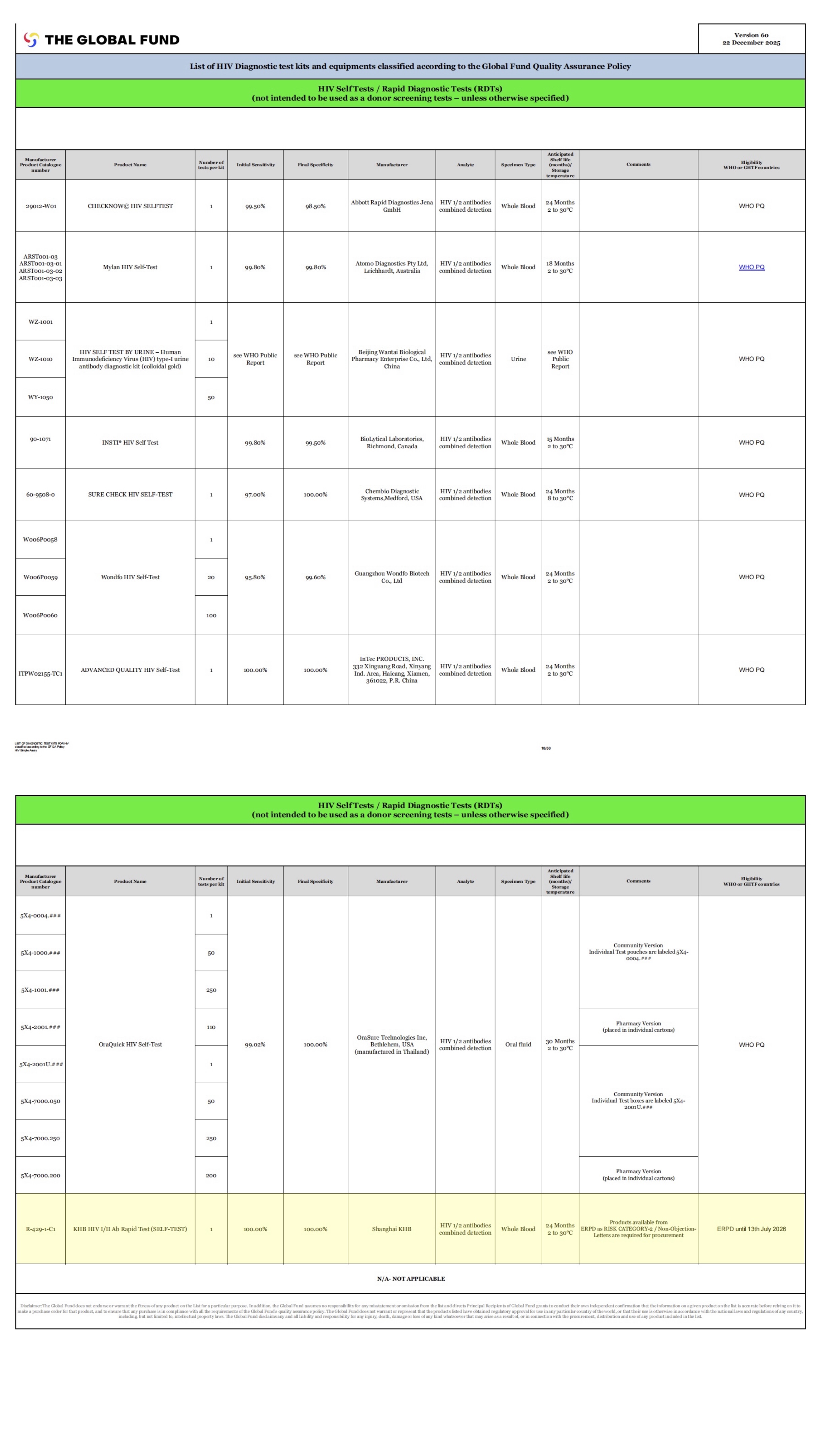
Shanghai Kehua Bio-engineering Co., Ltd. (KHB) is proud to announce that our KHB HIV I/II Ab Rapid Test (SELF-TEST) has recently been reviewed and granted Category-2 status by the Expert Review Panel for Diagnostics (ERPD), authorized by The Global Fund. This recognition positions KHB among the few Chinese companies included in the Global Fund’s procurement list.
What is ERPD and What Does it Mean for KHB's HIV Self-Test?
The Global Fund ERPD evaluates diagnostic products based on quality, effectiveness, and compliance with international standards, ensuring their essential role in addressing global public health emergencies. This process is particularly critical for enabling rapid responses in areas such as pandemic control, facilitating the swift accessibility and distribution of products by international health organizations.
As a fast-track mechanism, the ERPD reviews high-performing in vitro diagnostic (IVD) products that have not yet undergone WHO Prequalification (PQ). The evaluation, conducted by an independent expert panel, serves as a key reference for procurement decisions by international agencies and national governments, ensuring timely availability of effective diagnostics.
KHB’s HIV I/II Ab Rapid Test (SELF-TEST) was granted Category-2, enabling the product to be considered for temporary procurement through The Global Fund and UNITAID until 13th July 2026. This opens the door to broader access to vital diagnostics, especially in resource-limited settings, ultimately improving health outcomes globally.
Next Steps for KHB’s HIV Self-Test
The Category-2 status allows temporary procurement of our product, contingent on ad hoc review of procurement requests. In the coming months, we will collaborate closely with The Global Fund and UNITAID to meet necessary regulatory and quality requirements.
Our next major objective is to achieve WHO prequalification to expand the product’s global reach and accessibility.
Product Overview
The KHB HIV I/II Ab Rapid Test (SELF-TEST) is designed for the quick and reliable detection of HIV-1 and HIV-2 antibodies in serum, plasma, venous whole blood, and fingerstick whole blood.
Test Principle: Colloidal Gold Immunochromatography
Sensitivity: 99.82% (1103/1105)
Specificity: 100% (2051/2051)
Time to Result: 15-25 minutes
This rapid self-test offers accurate and fast results, providing individuals and healthcare providers with reliable information to support early HIV detection and decision-making.

Partner with Us
Since 2016, KHB has been a leader in global HIV diagnostics, with our professional HIV rapid test achieving WHO Prequalification (WHOPQ) and CE-IVDR certification in 2023. We collaborate with organizations such as WHO, UNICEF, USAID, and The Global Fund, with our products used in dozens of countries worldwide.
Whether you're a public health organization, procurement agency, or diagnostic distributor, KHB Group is ready to support large-scale deployments with reliable, high-quality solutions.
For procurement inquiries, please contact us at: international@skhb.com.
